The Martian Meteorite Compendium
Afterword
What should we be looking for?
Mars is, of course, a large planet with many features (mountains, craters, basins, volcanoes, lava flows, deep
graben, sand dunes, apparent river beds and deltas) indicating a complicated geological history. And Mars
is the future destination of future astronauts. The 32+ samples of Mars described in this Compendium give
us our first glimpse of what these future astronauts might find. Of course, we've also had the highly successful
Viking, Pathfinder and MER lander missions as well as Mars Surveyor and Odyssey orbiter missions.
Currently Mars Express and two MER missions with rovers are collecting data and more missions are
planned. The data from all these sources will need to be integrated into one history for Mars, and form basis
of the database for the "engineering model" required for detailed planning eventual human exploration.
Figure 1 presents a comparison of chemical composition obtained by X-ray fluorescence on Viking (Clark et al. 1982), APX on Pathfinder (Economou et al. 2003, Foley et al. 2003) and APX on the Spirit
and Opportunity Rovers (McSween et al. 2004) with data for Martian meteorites and some selected terrestrial basaltic and ultramafic rocks (diagram modified after Jagoutz et al. 1979). Note that all Martian samples have lower MgO and Al2O3 contents when compared with terrestrial samples (see McSween 2002). Another surprise is that the Martian meteorites are relatively reduced, with most of the Fe as +2, while the Martian surface is reddish, indicating that the surface materials are oxidized with much of the Fe in the +3 oxidation state (e.g. Morris et al. 2001). Martian meteorites are also relatively dry and unaltered considering that there is a lot of evidence for water at the Martian surface. Finally, we understand that all the cratering would have created a fragmental layer (called regolith) on Mars similar to that of the Moon. However, the Martian meteorites discovered so far, are all igneous rocks with only slight evidence of water and desiccated salts. There is only slight evidence that some of these rocks may contain small amounts of melted soil (Rao et al. 1998 and on).
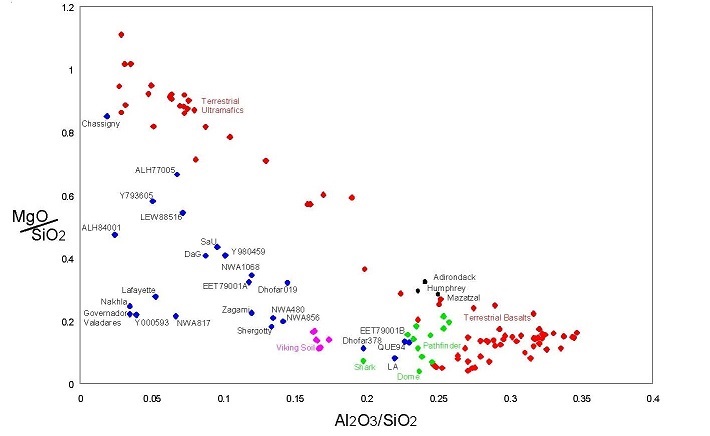
Legend Color Meaning Blue Martian Meteorite Green Pathfinder Pink Viking Black Mer Red Terrestial rocks Figure 1. Composition diagram comparing Martian Meteorites with Pathfinder (Table 1), Viking (Table 2) and MER mission data for
soils and rocks, and including data for terrestrial rocks (from Basaltic Volcanism of Terrestrial Planets, Tables 1.2.1.5a, 1.2.3.4, 1.2.3.6,
1.2.3.7, 1.2.4.1, 1.2.5.2 and 1.2.11.4).
Table 1 Chemical Composition of Mars Surface Materials by APX from Economou et al. 2003, McSween et al. 2004
Pathfinder rocks Pathfinder soils MER rocks Barnacle Yogi Wedge Shark Dome A-2 A-4 A-5 A-9 A-10 A-15 A-8 Adiron Humph Maza SiO2 53.9 45.7 47.2 51.5 50 40.9 41 41.7 41.7 41.2 43.2 44.6 45.4 46.1 45.7 TiO2 0.6 0.7 0.7 0.5 0.7 0.7 1 0.6 0.8 0.8 0.8 0.7 0.46 0.52 0.48 Al2O3 12.8 10.8 11.3 10.2 12.3 10.4 10.6 10.6 10.2 9.7 9.9 10.4 10.9 10.6 11.7 FeO 16.7 16.5 18.3 15.1 17.9 Fe2O3 21.2 20.4 21.8 22.2 23.9 23.2 18.9 3.02 2.99 1.8 MnO 0.3 0.4 0.3 0.4 0.4 0.5 0.4 0.2 0.1 0.4 0.3 0.3 0.41 0.39 0.36 CaO 5.7 6.3 6.8 7.3 6 6.1 5.6 6.2 6.4 6 5.5 6.9 7.49 7.7 7.71 MgO 2.1 5.1 4 3.7 3.4 8.7 8 7.3 6.4 7.5 6.7 6.3 12.8 12.2 11.6 Na2O 3.2 4.7 4.8 3.4 4 3.2 3.2 3.3 2.6 1.8 2.7 3.1 2.79 2.59 3.3 K2O 1.1 0.7 0.8 0.8 1 0.5 0.5 0.5 0.7 0.4 0.7 0.8 0.06 0.06 0.07 P2O5 0.7 0.5 0.5 0.4 0.6 0.9 1.2 0.6 0.8 0.6 0.6 0.5 0.52 0.56 0.7 Cr2O3 0.1 0.1 0 0.1 0.1 0.3 0.4 0.5 0.2 0.3 0.3 0.1 0.6 0.59 0.56 Cl 0.5 0.7 0.6 0.5 0.7 0.7 0.8 0.8 1.2 0.8 0.8 0.9 0.09 0.11 0.15 SO3 2 4.3 3 1.6 3 6 6.9 5.8 6.6 6.3 5.2 5.4 H2O* 0.3 3.4 1.5 4.3 0.1 0.3 1 total 100 99.9 99.8 99.8 100.1 100 99.9 * calculated based on excess oxygen assuming Fe+2 for rocks and Fe+3 for soils (Foley et al. 2003).
Table 2 - Chemical Composition from Viking (XRF) by Clark et al. 1982.
C-1 C-2 C-5 C-6 C-7 C-8 C-9 C-11 C-13 U-1 U-2 U-3 U-4 U-5 U-6 U-7 SiO2 43 42 42 44 44 43 45 43 42 43 44 44 43 42 42 TiO2 0.65 0.57 0.6 0.61 0.63 0.71 0.71 0.64 0.59 0.6 0.63 0.64 0.52 0.44 0.48 0.51 Al2O3 7.5 6.9 7.3 7.4 7.1 7.5 7.2 7 Fe2O3 17.6 17.3 17.4 17.3 19 18.8 18.9 17.7 18.2 18.9 17.6 18.3 16.9 16.3 17.1 17.5 CaO 6 5.5 5.6 6 6 5.8 6 5.4 5.4 5.8 5.8 5.95 5.7 5.3 5.5 5.5 MgO 6 7 6 5 6 5 6 7 K2O 0.04 0.03 0.02 Cl 0.7 0.7 0.9 0.8 0.6 0.65 0.8 0.9 0.3 0.6 0.45 0.6 0.3 0.4 SO3 7 9 9.5 6.7 6.8 5.9 7.2 9 8.4 8.1 7.6 7.9 8.3 7.9 7.6
CAUTION: The values listed in these tables have associated errors that are significant (see Economou et al. 2003, Clark et al. 1982).
At the rate we have been finding Martian meteorites (a few per year), we can expect a few more. What should we be looking for? Certainly more shergottites and nakhlites, but perhaps some other unique samples (such as; Chassigny, ALH84001). But what we really would like to find, is a sample of sedimentary or regolith material from Mars that could be used to understand the surface of Mars (Wright et al. 1994). So far, all of the Martian meteorites have been identified as igneous rocks and many are basalts. However, since many of the meteorites from the Moon are regolith breccias (Warren 2001), one can expect that we will also eventually obtain a Martian meteorite from the Martian surface!
Based on ejection ages (see figure I-6), it appears these first 32 samples were all derived from only a few (6 to 8) large craters (and we don't know where)! Since the impact has to be large enough to launch a meteorite off of Mars, this may explain the lack of regolith material. Either we only get the deep material from these impacts, or the surface material is friable and doesn't survive the shock, or we can't recognize a sample of the surface of Mars.
Since the surface material on Mars should be oxidized and contain substantial volatiles, one might sort through the Meteorite collections of the world for a reddish, frothy rock!
Science is knowledge gained by the study of the behavior of nature
While describing and studying Martian meteorites, scientists have learned to better understand important
geological processes such as; partial melting, crystallization, element partioning, diffusion, oxidation,
impact processes, shock levels, magma assimilation, etc. This knowledge will, in turn, prove useful understanding our planet!
Comparison of chemical and isotopic data from Martian meteorites tell us about the deep interior processes, such as; partial melting to form basalt (the shergottites), the nature of the depleted mantle, the oxidation state of the source regions, and even the initial core-mantle-crust segregation. Much of this knowledge is "model dependent", but the models are becoming better. These models also improve as terrestrial processes are better understood.
Martian meteorites (except for ALH84001) are relatively young, yet they have low initial isotopic ratios for Sr and Nd indicating that their igneous source regions are "depleted" in radioactive parent elements (Rb, Sm) (Borg et al. 1997). Many shergottites (e.g. ALH77005, QUE94201 etc.) also have REE patterns that show a strong depletion in LREE. It is not yet clear why the parts of the Martian mantle that are apparently "depleted" in the heat producing elements are melting to produce the relatively young basalts (1.3 to 0.2 billion years old).
Current research on Martian meteorites (2003) relates to an understanding of the oxidation state of the igneous rocks (Herd et al. 2002, 2003, Wadhwa 2001). These researchers find that the oxygen fugacity of Martian meteorites correlates with their initial Sr87/86, Nd143/144 and La/Yb ratios. This indicates (to them) that reduced magma produced in the Martian mantle becomes "contaminated" with oxidized, trace–element–rich, Martian crust as it ascends to the surface (which is the terrestrial experience as well).
Experimental studies are another very valid method to gain further knowledge of processes active on Mars. It has taken a lot of experiments to find the melt that would first crystallize pyroxenes with the compositions found in Martian meteorites (e.g. McKay et al. 1985 and on). Carefully–crafted experimental approaches to trace–element partioning have facilitated an understanding of the oxygen fugacity of the basaltic melt (Musselwhite and Jones 2002, 2003), the composition of Martian core (Righter and Shearer 2003) and the water content of basaltic magma (Dann et al. 2001). Phase equilibria studies have led to a better understanding of basalts (Stolper and McSween 1979, McCoy and Lofgren 1999) and the role of water in Martian magma systems (Mysen et al. 1998). Highpressure melting experiments that reproduce the basaltic compositions will lead to an understanding of the Martian mantle (e.g. Agee and Draper 2003). The effect of shock on the water and D/H contents of amphibole are being studied experimentally by Minitti et al. 2003. Inorganic, aqueous precipitation of Mg,Ca carbonates has shown that zoning patterns found in the carbonates in ALH84001 can be reproduced in the laboratory (Goldin et al. 2000 and on). Carefully planned, laboratory experiments such as these supplement and complement our understanding of these meteorites, and of Mars, and limit our otherwise wild speculation based on observations of the rocks alone.
Studies on terrestrial analogs to Martian meteorites would also contribute to an understanding of processes that form these samples. Some examples include: Lentz et al. (1999c) have studied the mineralogy of Theo's Flow with a goal of better understanding the origin of the nakhlites, Treiman et al. (2003) have studied carbonates in Spitzbergen and compared them to the "rosetts" in ALH84001, Newsom et al. have studied shocked basalts from Lonar crater and Morris et al. (2000) have studied altered basaltic soils for the top of Mona Kea. But clearly more studies of carefully chosen terrestrial analogs would be desirable (Harvey 2001).
Lessons Learned (a little knowledge is a dangerous thing)
- Planets can exchange materials (Melosh and Tonks 1993, Gladman et al. 1996). And this exchange must be significant over a period of 4 billion years. During the "cataclysm" 4 billion years ago, it must have been very significant!
- Mars differentiated early and has not been well–mixed since (Harper et al. 1995).
- Oxygen isotopes make a good discriminator of planetary bodies (Clayton and Mayeda 1996).
- The isotopic study of light elements is important on Mars. We don't know the initial ratios (starting points), but we do know that they evolved by significant loss of Martian atmosphere to space. Isotopic data need to be obtained concurrently for all light elements on aliquots of the same sample split. Additional isotopic exchange experiments need to be performed (Socki et al. 1993, Leshin et al. 1996, Jull et al. 1997).
- Some "maskelynite" is really plagioclase glass (Ikeda 1994, Stöffler et al. 1996, Scott et al. 1997).
- The least expensive way to obtain samples of Mars is to collect them as meteorites in Antarctica and/or in desert regions (Yanai 1997, Cassidy and Rancitelli 1982, Hofmann et al. 2003).
- The Public seem to be excited about the possibility of finding evidence of life on Mars (Kerr 1996, 1997).
- Samples studied in sophisticated terrestrial laboratories can obtain superior analytical data (Drake et al. 1987, Gooding et al. 1989). Data obtained by spacecraft are not precise and accurate enough to solve some important problems (e.g. age dating). In addition, in the laboratory, analyses can be performed over and over again, until optimum experimental design is achieved.
- The composition of the Martian atmosphere can best be determined by measuring the gasses released from glass inclusions in Martian meteorites(Bogard and Garrison 1998, Garrison and Bogard 1998).
- Martian samples, whether meteorite or to-bereturned by spacecraft, are best studied in "consortium mode", because data from various analytical techniques need to be performed on the same aliquot in order to obtain superior interpretation.
Unanswered questions (questions are more important than answers)
- If there is so much evidence for water at the surface of Mars, why do we see so little evidence for water in the Martian meteorites? (Baker 2001)
- If life existed on Mars, how was it different? (de Duve 1995)
- What is the composition of organic matter on Mars? (Wright et al. 1989, McKay et al. 1996, Becker et al. 1997)
- What was the history of the magnetic field on Mars and did Mars ever have a molten metal core? (Terho et al. 1996, Kirschvink et al. 1997, Collinson 1997, Stevenson 2001)
- What is the nature of interaction of fluids on Mars with the rocks? (Gooding 1992, Plumlee et al. 1993, Griffith et al. 1995, Jakosky and Phillips 2003)
- Is it possible to constrain the age of various terrain mapped on Mars, and thus establish an absolute time scale for Martian epochs? (Nyquist et al. 1997)
- How did the composition of the Martian atmosphere vary with time? (Wright et al. 1990a, Pepin 1994, Wright et al. 1996a)
- What is the nature of the Martian mantle (source region of basalts)? Does it contain garnet? Is it depleted in large-ion-lithophile elements? (Gleason et al. 1996, Jones et al. 1997)
- Which craters on Mars can be related to each of the groups of Martian meteorites? (Barlow 1997, Mouginis-Mark et al. 1992)
- Will the thermal emission spectroscopy experiment be able to distinguish and map rock types from orbit? (Hamilton et al. 1997, Christensen et al. 2003)
- If we already have 32 samples from Mars, why do we need MSR? This question can be answered, but this is not the place!
Water
"Water" on Mars has been said to be the unifying theme of NASA's Mars Surveyor Program. Edgett and Parker argue that "a vast portion of the Martian ancient cratered terrain was once under water". This is interesting in light of the carbonates found in the ancient meteorite, ALH84001 (chapter X) (Warren 1998, McSween et al. 1998).
Some water has been reported in Martian meteorites (Kerridge 1988, Karlsson et al. 1991, 1992, Leshin et al. 1996c). In addition, hydrous minerals have been reported (e.g. Treiman 1983). The phosphates do not seem to indicate that much water was present when magma crystallized, however, some phosphates have high a variable D/H ratios and seem to have reacted with the Martian atmosphere (Watson et al. 1994, Guan et al. 2003). The salts found in Martian meteorites have already given us a tantalizing clue about the nature of water on the surface of Mars (Gooding 1992, Warren 1998, Sawyer et al. 2000). The weathering product "iddingsite" found in Lafayette has been tentatively dated at about 700 Ma (Swindle et al. 1997, Shih et al. 1998).
The presence of up to 1.8% water is indicated in the source region for the basaltic magma for Shergotty (McSween et al. 2000, 2001).
Sample Return
Close–up photographs of rocks and soils taken by two Viking landers, the Pathfinder lander, Sojourner rover (and especially the two MER missions) have inspired the scientific community to evaluate what could be learned from a sample that might be returned from the surface of Mars (Brett 1974, Gooding 1990, Longhi 1996, Bogard 1996). Workshops have been held on Mars sample return science at the Johnson Space Center (Drake et al. 1987), in Europe (Jessberger 1991) and at the Ames Research Center (Gulick 1996). Numerous committees; MSHARP led by Mike Carr, MESG led by Dan McCleese (2001), MEPAG led by Ron Greeley (2001) and MSAG led by Glenn MacPherson (2001) have been charged with planning the requirements for sample return and precursor missions.
Rationale for sample return from Mars has been articulated by Bogard et al. (1979), Gooding et al. (1989), Allen and Treiman (1995), Allen (1996), Neal (1998) and others.
However, a lengthy report titled "An Exobiology Strategy for Mars Exploration" by Kerridge et al. (1995) generally overlooked the great potential of sample return and/or the careful study of Martian meteorites. Issues and recommendations related to the return of a Martian sample to Earth have been discussed by the National Academy of Science (Nealson et al. 1997) and others.
Scientific instruments carried on robotic missions are not precise and accurate enough to allow age determination, and/or geochemical modeling. For this, trace element ratios need to be determined accurately, and so far, this can only be done in highly sophisticated terrestrial laboratories. Analytical instrumentation has improved in response to the need for understanding Martian meteorites. Pillinger’s group has miniaturized mass spectrometers to handle small volumes of gas. Compston’s group has invented a whole new line of super-sensitive, high-resolution, ion microprobes to analyze small spots on thin sections. Wasserburg’s group and others have greatly improved analytical precision of isotopic ratio measurements. Zare’s group has invented laser desorption mass spectrometers. McKay et al., Bradley et al. and others are using the most advanced scanning electron microscopes to look for evidence of submicroscopic "nanofossils". And there are many other new instrumental techniques that have been utilized on rocks for the first time, in an effort to learn more about Mars by careful study of Martian meteorites.
International cooperation is clearly indicated in the exploration of Mars, and this has already begun with the study of Martian meteorites and with the MER misssions. Sample return by spacecraft has been long sought by this community of international scientists. Planning for a Mars Sample Return mission needs to include substantial technology development (Carr and Garvin 2001). Also see: http://www.jpl.nasa.gov/missions/future/mars2005andbeyond.html. In the meantime, careful research on Martian meteorites, helps us to further define the important questions, refines our tools and should influence the requirements for such a mission (Agee et al. 2000).
